Laser coding of pills
06/05/2020
Laser coding of pills
On other occasions we have seen examples of marking in the pharmaceutical industry to comply with the appropriate regulations corresponding to the FDA’s Unique Device Identification (UDI) or the European Union’s Falsified Medicines Directive (FMD). In this case, we go beyond the product packaging and directly mark the pills.
At Macsa id we have a clear understanding of the needs of the industry, which allows us to provide the optimal solution for each situation. In this case, the best tool for coding this type of product is laser marking. The laser ensures maximum control of both the marking characteristics and the properties obtained in the final product in a non-contact process, which guarantees that the product is not subjected to mechanical stress. This guarantees the hygiene and biological compatibility of the marking produced with the medicine’s functionality.
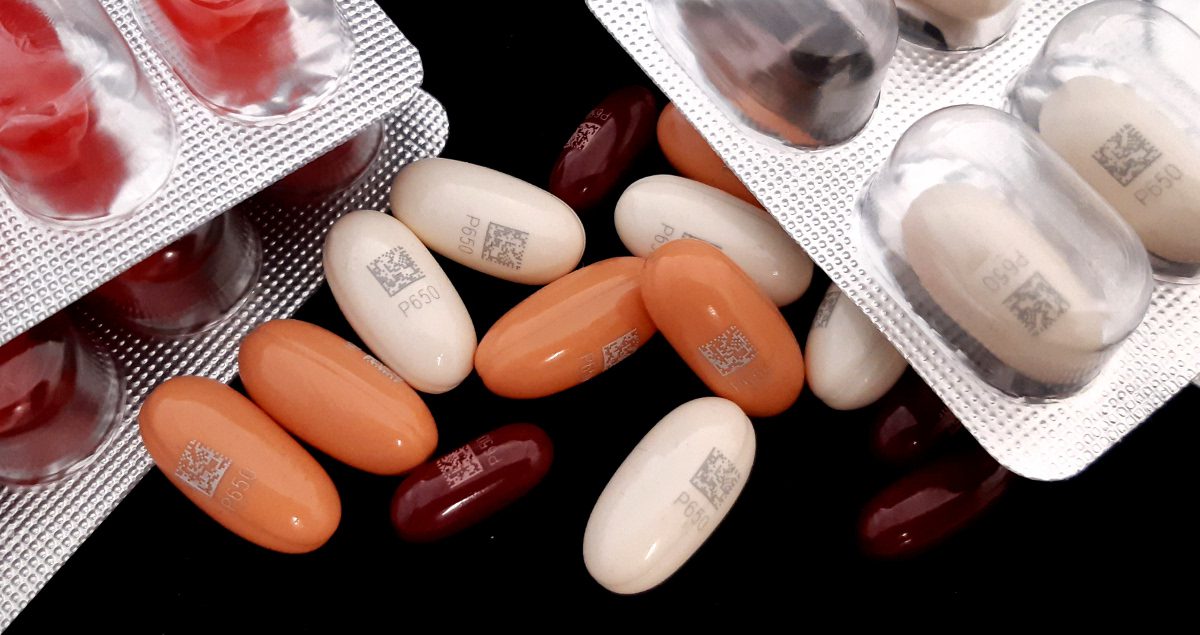
These pills, formed with a mixture of gelatine and titanium dioxide, use different dyes to better differentiate them. This causes a wide variety of colours to be marked, which affects the choice of the best marking system. In our case, by means of a DPSS equipment with emission in green (532 nm) we are able to obtain a high definition and easily readable marking for the whole range of colours. High speed marking, with a time of 150 milliseconds per capsule, ensures correct coding for high production situations in the laboratory.
Technical information
- Laser: D-5005-Green
- Lens: 100×100
- Market: Pharma
- Application type: Coding
- Product: Pills
- Material: Gelatine and titanium dioxide
- Marking type: Static
- Marking time: 0.05 seconds
If you need more information about laser coding of pills, don’t hesitate to contact us.


